Highlights
STMC Research Highlights
Pre-assembled GPCR signaling complexes mediate unique cellular responses to ultralow ligand concentrations
G protein–coupled receptors (GPCRs) are the largest class of cell surface signaling proteins, participate in nearly all physiological processes, and are the targets of 30% of marketed drugs. Typically, nanomolar to micromolar concen- trations of ligand are used to activate GPCRs in experimental systems. We detected GPCR responses to a wide range of ligand concentrations, from attomolar to millimolar, by measuring GPCR-stimulated production of cyclic adenosine monophosphate (cAMP) with high spatial and temporal resolution. Mathematical modeling showed that femtomolar concentrations of ligand activated, on average, 40% of the cells in a population provided that a cell was activated by one to two binding events. Furthermore, activation of the endogenous β2-adrenergic receptor (β2AR) and muscarinic acetylcholine M3 receptor (M3R) by femtomolar concentrations of ligand in cell lines and human cardiac fibroblasts caused sustained increases in nuclear translocation of extracellular signal–regulated kinase (ERK) and cytosolic protein kinase C (PKC) activity, respectively. These responses were spatially and temporally distinct from those that occurred in response to higher concentrations of ligand and resulted in a distinct cellular proteomic profile. This highly sensitive signaling depended on the GPCRs forming preassembled, higher-order signaling complexes at the plasma membrane. Recognizing that GPCRs respond to ultralow concentrations of neurotransmitters and hormones challenges established paradigms of drug action and provides a previously unappreciated aspect of GPCR activation that is quite distinct from that typically observed with higher ligand concentrations.
Civciristov S, Ellisdon AM, Suderman R, Pon CK, Evans BA, Kleifeld O, Charlton SJ, Hlavacek WS, Canals M, Halls ML (2018) Pre-assembled GPCR signaling complexes mediate unique cellular responses to ultralow ligand concentrations. Sci Signal 11: eaan1188.
ROCK regulates the intermittent mode of interstitial T cell migration in inflamed lungs
T cells play and important role in the protection against infections and cancer, but they can also cause disease by mediating inflammatory tissue damage. Lung inflammation is a major clinical problem, but we know only very little of how the in situ behavior of T cells promotes an inflammatory environment. In this study, we used a murine model of acute lung injury to investigate migration and interaction of lung-infiltrating T cells, with a combination of experiments and computational analysis. Using two-photon imaging we directly visualized T cells in live inflamed lung. We found that some T cells moved very actively and other T cells appeared non-motile.
With the support of the STMC, we developed computational approaches to reveal that T cells display a novel intermittent migration pattern, switching between straight migration and confined motion. T cell move using blood vessels for guidance. T cell movement also requires ROCK, a regulator of the cytoskeleton. Unexpectedly, chemokines are less important for intermittent T cell movement. We then used computer simulations to test the rationale for why T cells show intermittent confinement. Our simulations show that the intermittent T cell migration pattern promotes long-lasting stable contacts between T cells and target cells, so that it becomes less likely that T cells “overlook” relevant targets. In conclusion, this study identifies a novel mechanism that balances thoroughness and extensiveness of the search behavior of lung-infiltrating T cells.
Mrass, P., Oruganti, S.R., Fricke, G.M., Tafoya, J., Byrum, J.R., Yang, L., Hamilton, S.L., Miller, M.J., Moses, M.E., and Cannon, J.L. (2017). ROCK regulates the intermittent mode of interstitial T cell migration in inflamed lungs. Nat Commun 8, 1010.
Timescale Separation of Positive and Negative Signaling Creates History-Dependent Responses to IgE Receptor Stimulation
The high-affinity receptor for IgE expressed on the surface of mast cells and basophils interacts with antigens, via bound IgE antibody, and triggers secretion of inflammatory mediators that contribute to allergic reactions. To understand how past inputs (memory) influence future inflammatory responses in mast cells, a microfluidic device was used to precisely control exposure of cells to alternating stimulatory and non-stimulatory inputs. We determined that the response to subsequent stimulation depends on the interval of signaling quiescence. For shorter intervals of signaling quiescence, the second response is blunted relative to the first response, whereas longer intervals of quiescence induce an enhanced second response. Through an iterative process of computational modeling and experimental tests, we found that these memory-like phenomena arise from a confluence of rapid, short-lived positive signals driven by the protein tyrosine kinase Syk; slow, long-lived negative signals driven by the lipid phosphatase Ship1; and slower degradation of Ship1 co-factors. This collaborative work, by investigators at Sandia National Laboratories, Los Alamos National Laboratory, Cornell and UNM, advances our understanding of mast cell signaling and represents a generalizable approach for investigating the dynamics of signaling systems.
B Harmon, LA Chylek, YLiu, E D Mitra, A Mahajan, EA Saada, BR Schudel, DA Holowka, BA Baird, BS Wilson, WS Hlavacek and AK Singh (2017) Scientific Reports 7:15586.
Figure caption: Mast cell degranulation responses depend on the history of multivalent antigen exposure. Response to a second exposure is attenuated relative to the first exposure if the two exposures are separated by a short period of IgE receptor signaling quiescence brought about by excess monovalent antigen. In contrast, the second response is amplified if the period of signaling quiescence is long.
Differential mast cell outcomes are sensitive to FcεRI-Syk binding kinetics
Crosslinking of IgE-bound FcεRI triggers multiple cellular responses, including degranulation and cytokine production. Signaling is dependent on recruitment of Syk via docking of its dual SH2 domains to phosphorylated tyrosines within the FcεRI immunoreceptor tyrosine-based activation motifs. Using single molecule imaging in live cells, we directly visualized and quantified the binding of individual mNeonGreen-tagged Syk molecules as they associated with the plasma membrane after FcεRI activation. We found that Syk colocalizes transiently to FcεRI and that Syk-FcεRI binding dynamics are independent of receptor aggregate size. Substitution of glutamic acid for tyrosine between the Syk SH2 domains (SykY130E) led to an increased Syk-FcεRI off-rate and impaired downstream signaling. These results highlighting the importance of finely-tuned protein-protein interactions in directing cellular outcomes.
S.L. Schwartz, C. Cleyrat, M. Olah, P. Relich, G. Phillips, W.S. Hlavacek, K.A. Lidke, B.S. Wilson and D.S. Lidke. “Differential mast cell outcomes are sensitive to FcεRI-Syk binding kinetics.” In press at Molecular Biology of the Cell – Quantitative Biology Special Issue. doi: 10.1091/mbc.E17-06-0350
**Selected by editorial board as a “Highlight from MBoC”
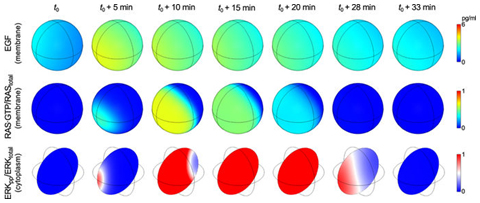
Modeling spatiotemporal kinetics of the Ras-MAPK cascade
To investigate spatial heterogeneities in MAP kinase signaling downstream of EGFR and RAS, Lipnaicki and STMC collaborator William Hlavacek developed a reaction–diffusion PDE-based model for RAS-RAF-MEK-ERK signaling. The model accounts for negative and positive feedback loops, the spatiotemporal kinetics of the signaling network components within the cell, and extracellular EGF. The PDE model was used to predict responses to localized (paracrine) EGF stimulation. A sequence of simulation snapshots is shown in the figure. This sequence illustrates how non-uniform EGF stimulation, which is visualized in the top row of images, can trigger a traveling wave of activated RAS that spreads over the plasma membrane and that produces a transient gradient of ERK activity within the cell, which is visualized in the bottom row of images. This spatiotemporal behavior is in accordance with local excitation-global inhibition dynamics.
Kochańczyk M, Kocieniewski P, Kozłowska E, Jaruszewicz-Błonska J, Sparta B, Pargett M, Albeck JG, Hlavacek WS, Lipniacki T (2017) Relaxation oscillations and hierarchy of feedbacks in MAPK signaling. Sci Rep 7: 38244. PMCID: PMC5206726
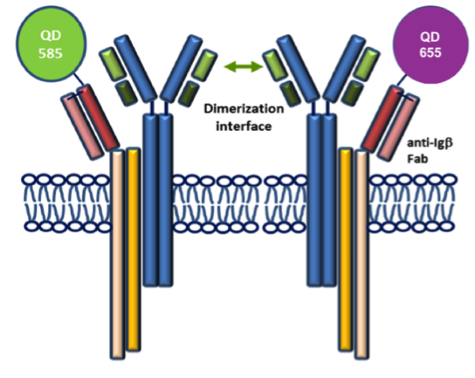
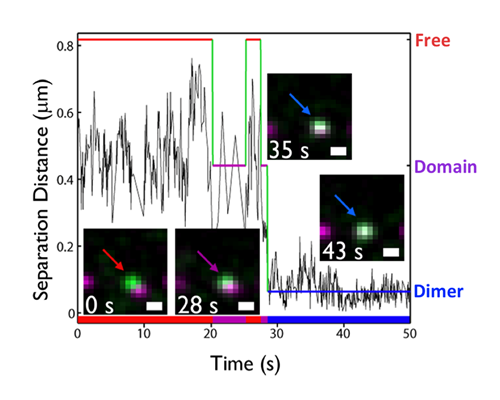
Dynamic pre-BCR homodimers fine-tune autonomous survival signals in B cell precursor acute lymphoblastic leukemia
STMC trainee Frank Erasmus and mentors Wilson and Lidke teamed up for this recent publication in Science Signaling. The pre-BCR is composed of membrane bound heavy chain (Igμ), surrogate light chain components and the signaling subunits, Igα/β. Here, monovalent Quantum Dot (QD)-labeled probes against Igβ were used for single particle tracking of pre-BCR engaged in autonomous signaling on live cells. Studies revealed that pre-BCRs engage in transient but frequent homotypic interactions. Motion was correlated at short separation distances, consistent with formation of dimers and occasional larger order oligomers. Repeated encounters between diffusing pre-BCRs reflects transient co-confinement in membrane domains. In the setting of acute lymphoblastic leukemia, these investigators showed that frequent, short-lived homodimers drive blast cell survival signals, including BCL6 expression. Pro-survival signaling from the pre-BCR can be blocked by inhibitory Fabs directed at surrogate light chain components or by tyrosine kinase inhibitors that block activity of two key downstream signaling partners, Lyn and Syk. By comparison, binding of dimeric galectin-1 results in large, highly immobile pre-BCR aggregates that are relieved in part by the addition of lactose to prevent crosslinking to other glycosylated membrane components. The pre-BCR and its signaling partners are being explored in the Wilson laboratory as potential therapeutic targets in precursor B-lineage acute lymphoblastic leukemia (BCP-ALL).
F Erasmus, K Matlawska, A Mahajan, SS Winter, L Xu, M Horowitz, DS Lidke and BS Wilson. (2016) “Dynamic pre-BCR homodimers fine tune autonomous signals for B lineage ALL survival.” Science Signaling, 9(456): ra116
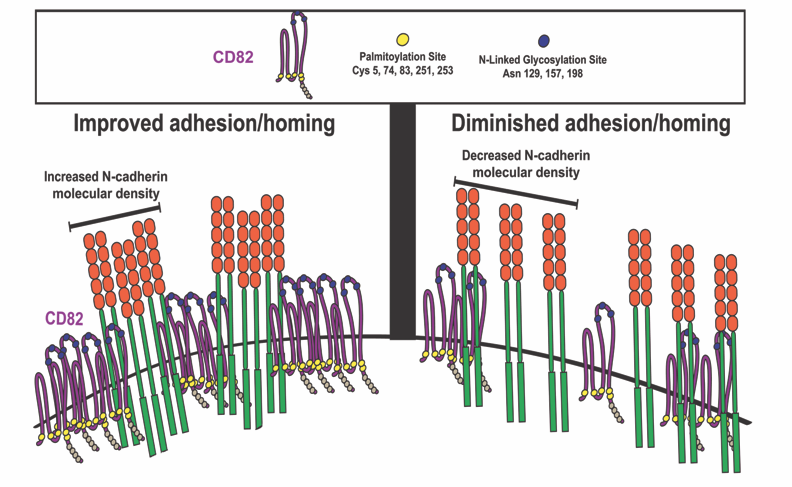
Tetraspanin CD82 regulates bone marrow homing of acute myeloid leukemia by modulating the molecular organization of N-cadherin
In this Oncogene paper, Jennifer M. Gillette, PhD and colleagues demonstrated that differential expression of the membrane scaffold CD82 modulates the bone marrow homing of Acute Myeloid Leukemia (AML) cells (Marjon KD, Termini CM, Karlen KL, Saito-Reis C, Soria CE, Lidke KA, Gillette JM. "Tetraspanin CD82 regulates bone marrow homing of acute myeloid leukemia by modulating the molecular organization of N-cadherin," Oncogene 2015, online November). By combining mutational analysis and super-resolution imaging, membrane protein clustering by CD82 was identified as a regulator of AML cell adhesion and bone marrow homing. Cluster analysis of super-resolution data (with STMC member Keith Lidke) indicated that N-linked glycosylation and palmitoylation of CD82 are both critical modifications that control the microdomain organization of CD82 as well as the nanoscale clustering of associated adhesion protein, N-cadherin. Inhibition of CD82 glycosylation was demonstrated to increase the molecular packing of N-cadherin and promote the bone marrow homing of AML cells. In contrast, inhibition of CD82 palmitoylation disrupeds the formation and organization of N-cadherin clusters and significantly diminished bone marrow trafficking of AML. Taken together, these data establish a mechanism where the membrane organization of CD82, through specific post-translational modifications, regulates N-cadherin clustering and membrane density to impact the in vivo trafficking of AML cells. These observations provide an alternative model for targeting AML where modulation of protein organization within the membrane may be an effective therapy to disrupt the bone marrow homing potential of AML cells.
Marjon KD, Termini CM, Karlen KL, Saito-Reis C, Soria CE, Lidke KA, Gillette JM. (2016) "Tetraspanin CD82 regulates bone marrow homing of acute myeloid leukemia by modulating the molecular organization of N-cadherin," Oncogene 35(31):4132-40.
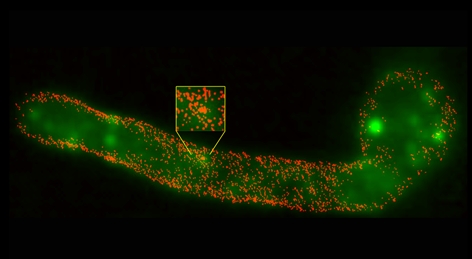
Unmasking of fungal β-glucan for improved dendritic cell phagocytosis
The cell wall of Candida albicans is composed largely of polysaccharides. In this new paper from the laboratory of STMC faculty member Aaron Neumann, the complexity of β-glucan as a major ligand for the immunoreceptor Dectin-1 is considered. The exposure of this cell wall polysaccharide is often restricted, or "masked", from immune recognition dendritic cells (DCs) and other innate immune cells. This team applied direct Stochastic Optical Reconstruction Microscopy (dSTORM) in the STMC SR Core to explore the fine structure of β-glucan exposed on C. albicans cell walls before and after treatment with the antimycotic drug caspofungin, which alters glucan exposure. They found that most surface-accessible glucan on C. albicans yeast and hyphae are limited to isolated Dectin-1 binding sites. Caspofungin-induced unmasking caused an ∼4-7 fold increase in total glucan exposure, accompanied by increased phagocytosis efficiency of DCs for unmasked yeasts. Nanoscopic imaging of caspofungin-unmasked C. albicans cell walls revealed that the increase in glucan exposure is due to increased density of glucan exposures and increased multi-glucan exposure sizes. These findings reveal that glucan exhibits significant nano-structure, which is a previously unknown physical component of the host-Candida interaction that may change during antifungal chemotherapy and impact innate immune activation. See also the Tools page for image analysis techniques used in this paper. Image: Super resolved fluorescence imaging reveals the nanoscopic structure of C. albicans hyphal glucan exposures (red) which are not resolved by conventional fluorescence imaging (green). Credit: Jia Lin, PhD
J Lin, MJ Wester, MS Graus, KA Lidke and AK Neumann (2016) Nanoscopic cell wall architecture of an immunogenic ligand in Candida albicans during antifungal drug treatment. Molecular Biology of the Cell. 27(6):1002-14.
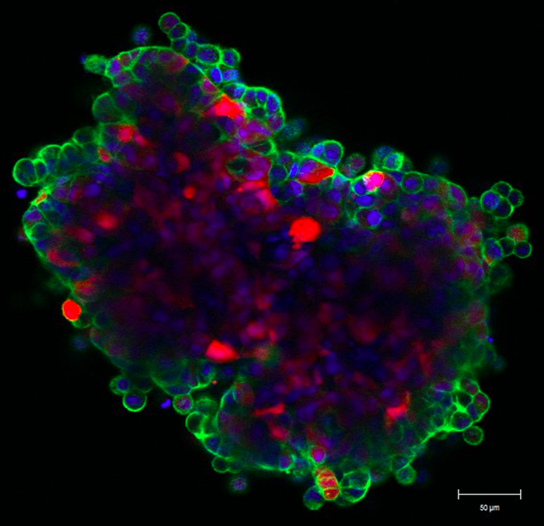
Mathematical modeling of drug delivery route for treatment of ovarian cancer
This STMC project describes the use of a cellular Potts model to evaluate tumor penetration of two classes of drugs (cisplatin and pertuzumab) when delivered by two alternative routes. The model was built upon in vitro and in vivo experimental data for drug delivered IV (intravenously) or IP (intraperitoneally), as well as vascular density in tumors. The model considers the primary route of drug administration, as well as the subsequent exchange into the other delivery volume as a secondary route. By accounting for these dynamics, the model revealed that intraperitoneal infusion is the markedly superior route for delivery of both small-molecule and antibody therapies into microscopic, avascular tumors typical of patients with ascites. Small tumors attached to peritoneal organs, with vascularity ranging from 2% to 10%, also show enhanced drug delivery via the intraperitoneal route, even though tumor vessels can act as sinks during the dissemination of small molecules. The model suggests that the most effective therapeutic route for targeting avascular and microscopic vascular ovarian tumors following debulking is likely to be peritoneal delivery. As secondary tumor volume expands, the use of both delivery routes is optimal to reach both avascular tumor spheroids common in ascites settings as well as neo vascularized tumors attached to peritoneal organs. Confocal image at left shows the "wave front" of fluorescent anti-erbB2 antibodies after 6 hr incubation with ovarian cancer spheroid (Steinkamp). Green: pertuzumab, Red: tumor cells, Blue: cell nuclei
KR Kanigel Winner, MP Steinkamp, RJ Lee, M Swat, CY Muller, ME Moses, Y Jiang, and BS Wilson. (2016) "Spatial Modeling of Drug Delivery Routes for Treatment of Disseminated Ovarian Cancer" Cancer Research 76(6):1320-34.
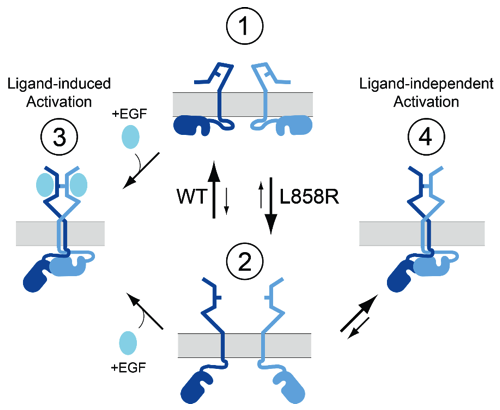
Enhanced dimerization drives ligand-independent activity of mutant epidermal growth factor receptor in lung cancer
This report characterizes the molecular mechanisms that drive oncogenic activity of lung cancer-associated mutant EGFR. STMC Project 2 PI, Diane Lidke, used high-resolution microscopy methods, including single quantum dot tracking, super-resolution and FRET-FLIM, to reveal that mutations within the kinase domain induce structural changes in the receptor extracellular domain via "inside-out" signaling. This altered structural equilibrium leads to enhanced dimerization of mutated receptors in the absence of ligand, which underlies unregulated signaling and oncogenesis. Patients harboring these mutations - L858R (or L834R) and exon 19 deletions - respond to clinical tyrosine kinase inhibitors, however relapse inevitably occurs due to acquired drug resistance. Lidke's results suggest that disrupting EGFR dimerization may offer a strategic therapeutic advantage, either alone or as a combination therapy with existing tyrosine kinase inhibitors.
C.C. Valley, D.J. Arndt-Jovin, N. Karedla, M.P. Steinkamp, A. Chizhik, W.S. Hlavachk, B.S. Wilson, K.A. Lidke, D.S. Lidke. (2015) "Enhanced dimerization drives ligand-independent activity of mutant EGFR in lung cancer." Molecular Biology of the Cell. 26: 4087-99 (2015)
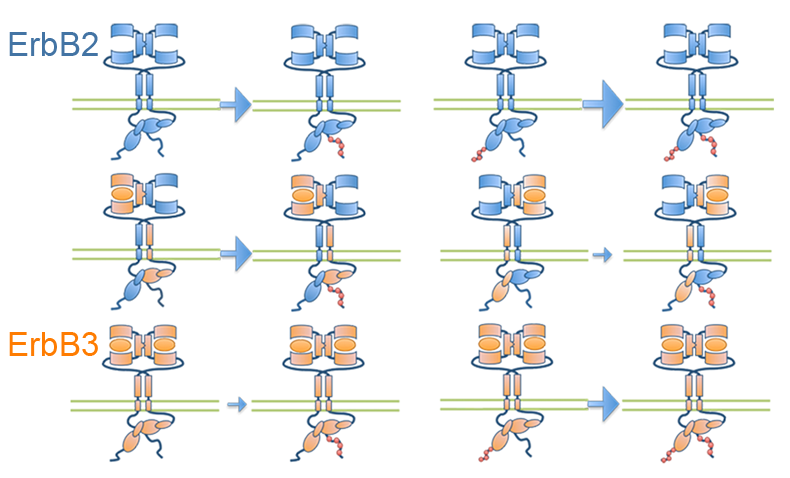
Orchestration of ErbB3 signaling through hetero and homo-interactions
Published in Molecular Biology of the Cell, STMC investigators have developed a spatial stochastic model that addresses the dynamics of ErbB3 homodimerization and ErbB2/ErbB3 heterodimerization. The model is based upon experimental measures for diffusion, dimer off-rates, kinase activity and dephosphorylation. Receptor confinement zones within the simulation landscape were reconstructed based on a new method that extracts spatial data from dynamic single particle tracking experiments. Simulations show the complex interaction between ErbB2 and ErbB3 as they cycle between monomeric and dimeric states. Computational analysis of ErbB3 mutations, predicts that activating mutations in the intracellular and extracellular domains may be subdivided into classes with distinct underlying mechanisms. Characterization of one ErbB3 gain-of-function point mutation located in the C-lobe asymmetric dimerization interface shows enhanced phosphorylation at low ligand doses and is associated with increased kinase activity.
Meghan McCabe Pryor, Mara P. Steinkamp, Adam M. Halasz, Ye Chen, Shujie Yang, Marilyn S. Smith, Gergely Zahoransky-Kohalmi, Mark Swift, Xiao-Ping Xu, Dorit Hanien, Niels Volkmann, Diane S. Lidke, Jeremy S. Edwards, and Bridget S. Wilson. (2015) Orchestration of ErbB3 signaling through hetero and homo-interactions. Mol. Biol. Cell mbc.E14-06-1114; First Published on September 16, 2015; doi:10.1091/mbc.E14-06-1114
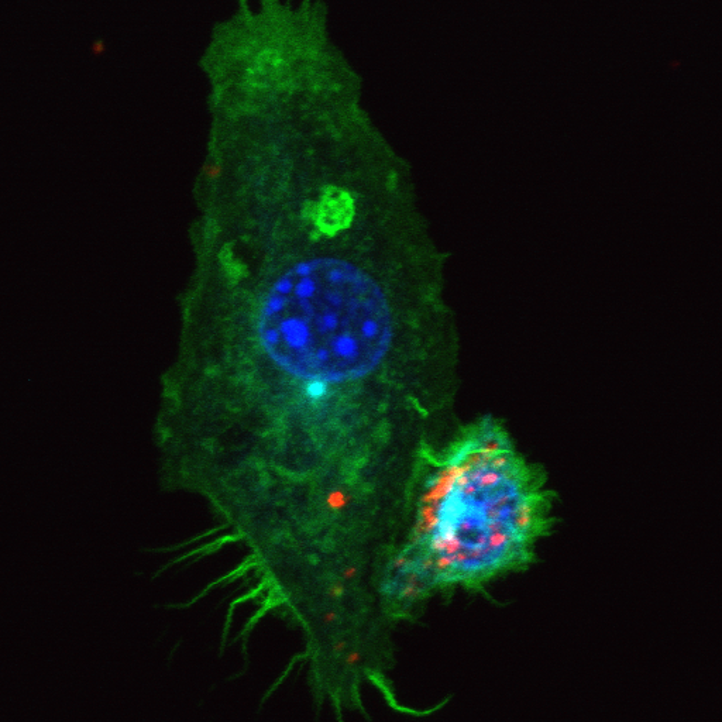
Mast cells and dendritic cells form synapses that facilitate antigen transfer for T cell activation
In this JCB article, STMC's Diane Lidke (senior author) and Judy Cannon, along with Alessandra Cambi, Amanda Carroll-Portillo, and colleagues showed that mast cells connect to dendritic cells before delivering antigens that can induce a response from T cells. When inactivated mast cells make contact with a dendritic cell, their interaction lasts only a few seconds. In contrast, an activated mast cell can remain attached to a dendritic cell for several minutes and this interaction is dependent on integrin engagement.
Amanda Carroll-Portillo, Judy L. Cannon, Joost te Riet, Anna Holmes, Yuko Kawakami, Toshiaki Kawakami, Alessandra Cambi, and Diane S. Lidke (2015) Mast cells and dendritic cells form synapses that facilitate antigen transfer for T cell activation. J. Cell Biol 210(5):851-64.
See also "In Focus" article: http://jcb.rupress.org/content/early/2015/08/19/jcb.2105if
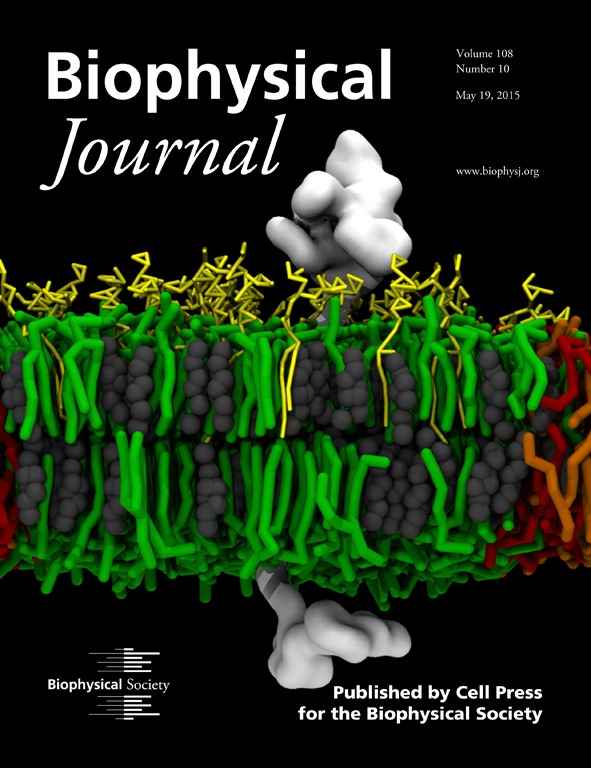
Membrane-Mediated Regulation of the Intrinsically Disordered CD3ϵ Cytoplasmic Tail of the TCR
Featured on the cover of Biophysical Journal: STMC investigators probed the molecular aspects of the interaction between a membrane and a signaling subunit (CD3ϵ chain) of the T-cell receptor (TCR) with the intention of shedding light on the potential role of membrane in the early events following the engagement of the TCR with the peptide-bound major histocompatibility complex. Signaling is initiated by the phosphorylation of the canonical tyrosines in the immunoreceptor tyrosine-based activation motifs (ITAMs) in the cytoplasmic tails of the signaling subunits. We used molecular-dynamics simulations to study the influence of different lipid compositions on the association between the disordered cytoplasmic tail and the inner leaflet of the bilayer, as well as the preferential partitioning of the CD3ϵ chain (white). In addition to predicting that the negatively charged lipid headgroup increases the association between the disordered tail and the inner leaflet, simulations predict that the CD3ϵ chain can discriminate between saturated (green) and unsaturated (orange, negatively charged; red, neutral) lipids found predominantly in the lipid-ordered (Lo) and lipid-disordered (Ld) domains, respectively. In systems mimicking biological membranes (yellow, gangliosides; gray, cholesterol), CD3ϵ chain partitioning is also modulated by gangliosides, revealing a plausible effect on activation through the regulation of lipid composition in cell membranes. On the basis of our findings, we propose that our model for the preferential colocalization and modulation of the CD3ϵ chain between the Lo-Ld phases may contribute to the underlying mechanisms for the activation of the ITAM-containing disordered chains in important immune receptors (e.g., T- and B-cell receptors).
Cesar A. López, Anurag Sethi, Byron Goldstein, Bridget S. Wilson, S. Gnanakaran. (2015) Membrane-Mediated Regulation of the Intrinsically Disordered CD3ϵ Cytoplasmic Tail of the TCR. Biophysical Journal 108(10):2481-91
Cell Membrane Nanodomains: From Biochemistry to Nanoscopy
This new book edited by STMC's Diane Lidke and collaborator Alessandra Cambi describes recent advances in our understanding of membrane organization. There is a particular focus on the cutting-edge imaging techniques that are making these new discoveries possible. With contributions from pioneers in the field, the book explores areas where the application of these novel techniques reveals new concepts in biology. It assembles a collection of works where the integration of membrane biology and microscopy emphasizes the interdisciplinary nature of this exciting field.
The book is out now on CRC Press.
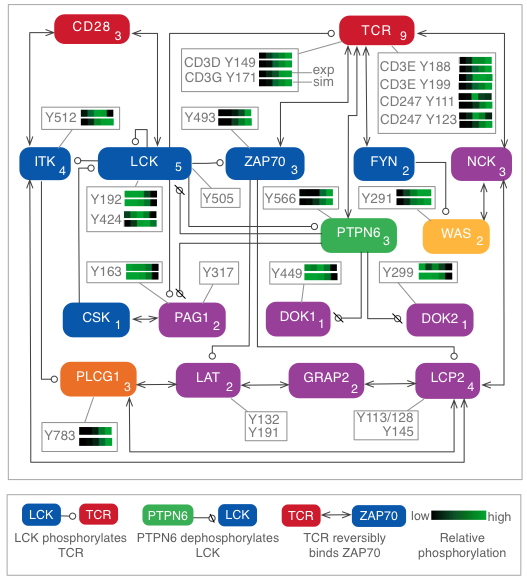
Quantitative proteomics and dynamical modeling elucidate missing links in the T-cell receptor signaling network
Stimulation of the T-cell receptor (TCR) can trigger a cascade of biochemical signaling events with far-reaching consequences for the T cell, including changes in gene regulation and remodeling of the actin cytoskeleton. A driving force in the initiation of signaling is phosphorylation and dephosphorylation of signaling proteins. This process has been difficult to characterize in detail because phosphorylation takes place rapidly, on the timescale of seconds, which can confound efforts to decode the order in which events occur. In addition, multiple residues in a protein may be phosphorylated, each involved in distinct regulatory mechanisms, necessitating analysis of individual sites. To characterize the dynamics of site-specific phosphorylation in the first 60 seconds of TCR signaling, we stimulated cells for precise lengths of time using a quench-flow system and quantified changes in phosphorylation using mass spectrometry-based phosphoproteomics. We developed a computational model that reproduced experimental measurements and generated predictions that were validated experimentally. We found that the phosphatase SHP-1, previously characterized primarily as a negative regulator, plays a positive early role in signaling by dephosphorylating negative regulatory sites in other proteins. We also found that the actin regulator WASP is rapidly activated via a shortcut pathway, distinct from the longer pathway previously considered to be the main route for WASP recruitment. Through iterative experimentation and model-based analyses, we have found that early signaling may be driven by transient mechanisms that have been overlooked in studies focused on later time points in signaling.
L. A. Chylek,* V. Akimov,* J. Dengjel,* K. T. G. Rigbolt, B. Hu, W. S. Hlavacek and B. Blagoev (2014) Phosphorylation site dynamics of early T-cell receptor signaling. PLOS ONE. 9(8):e104240..
*These authors contributed equally.
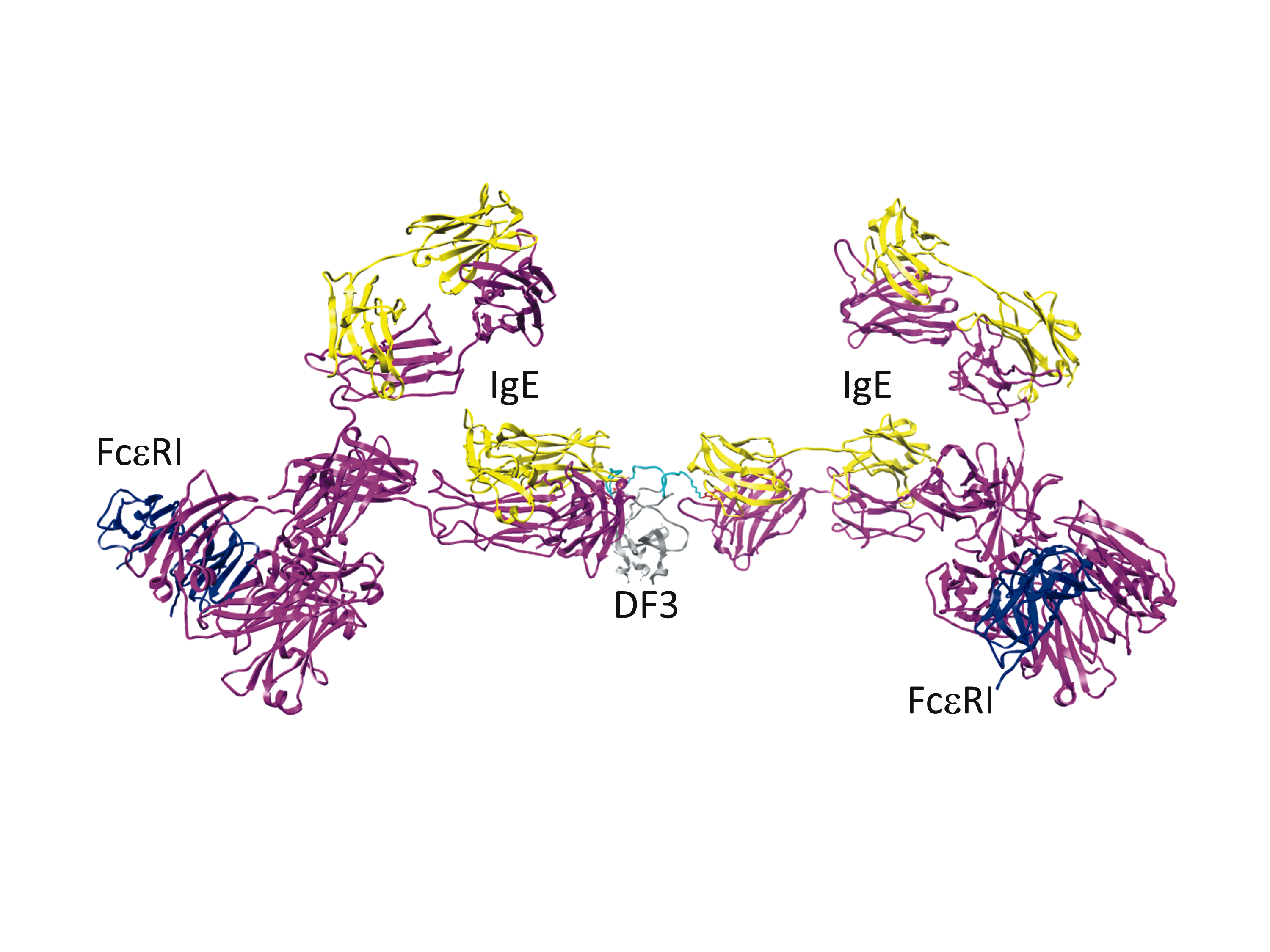
Mast cell stimulation with a novel trivalent ligand characterizes the nonlinear relationship between ligand dose, receptor aggregation and cell response
Aggregation of the high affinity IgE receptor (FceRI) by allergens triggers mast cell signaling that results in degranulation and release of mediators that constitute an allergic reaction. An interdisciplinary STMC team characterized a structurally-defined trivalent ligand (DF3) to stimulate mast cell responses using modeling and experimental approaches. Receptor aggregates were shown to form even at low and high DF3 doses where degranulation responses are poor. Reduced signaling outcome was linked to the rapid recruitment of negative regulatory inositol phosphatase SHIP, in addition to reduced positive signaling. Result demonstrate that optimal secretory responses result from a fine balance of positive and negative signaling in mast cells. This balance depends on the quality of receptor aggregates that promote sufficient positive signaling by Syk to override phosphatase-mediated negative regulatory signals. Delineating mechanisms of dose-dependent ligand induced mast cell responses is important for designing strategies to control allergic responses.
Avanika Mahajan, Dipak Barua, Patrick Cutler, Diane S. Lidke, Flor A. Espinoza, Carolyn Pehlke, Rachel Grattan, Yuko Kawakami, Chang-shung Tung, Andrew R. Bradbury, William S. Hlavacek, Bridget S. Wilson (2014) Optimal Aggregation of FcεRI with a Structurally Defined Trivalent Ligand Overrides Negative Regulation Driven by Phosphatases. ACS Chem Biol. Epub ahead of print. PMID 24784318
Getting to know you: Innate immune sensing of fungal pathogen surfaces
From the cover of PLOS Computational Biology, May 2014.
The earliest events in host defense against Candida species fungal pathogens involve the recruitment of anti-fungal receptors to specialized cellular contact sites. Image bioinformatic approaches developed at the STMC permitted quantitative dissection of spatiotemporal receptor distributions along this complex interface. Human immature dendritic cells (foreground right) recruit DC-SIGN (green) and CD206 (red) to capture C. albicans yeast (yellow), achieving up to two orders of magnitude enrichment of these receptors at the contact site.
Graus MS, Pehlke C, Wester MJ, Davidson LB, Steinberg SL, Neumann AK. (2014) A New Tool to Quantify Receptor Recruitment to Cell Contact Sites during Host-Pathogen Interaction. PLoS Comp Biol. 2014;10(5). PMID: 24874253. PMCID: PMC4038466.
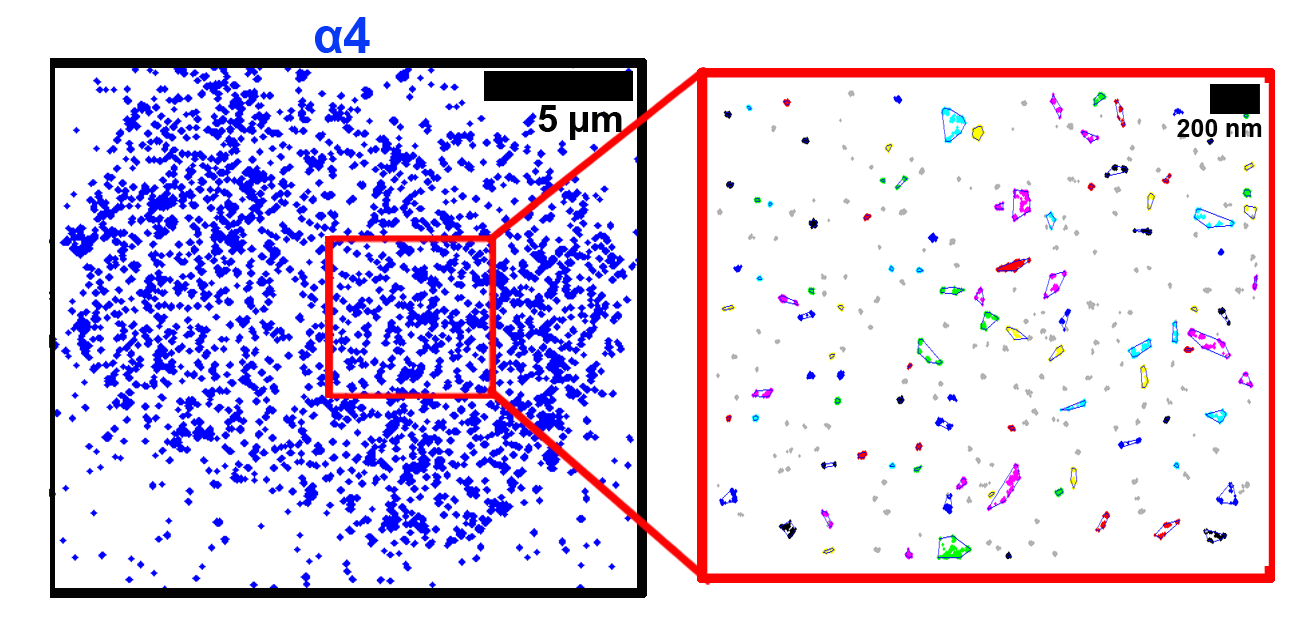
The membrane scaffold CD82 regulates cell adhesion by altering α4 integrin stability and molecular density
We have previously identified the tetraspanin protein, CD82, as a critical regulator of hematopoietic stem/progenitor cell (HSPC) adhesion and homing to the bone marrow. In this article, we combine the super-resolution microscopy technique, direct stochastic optical reconstruction microscopy (dSTORM) with clustering algorithms to investigate the role of CD82 and its palmitoylation in regulating the organization of CD82 and the α4 integrin subunit. We show visually and quantitatively that the palmitoylation sites within CD82 are critical for the organization of CD82, and also contribute to the tight packing of α4 integrins. These data support a model where CD82 clustering promotes increased α4 molecular density, which contributes to enhanced HSPC adhesion to extracellular matrix components.
Termini, C. M., Cotter, M. L., Marjon, K. D., Buranda, T., Lidke, K. A., Gillette, J. M. The membrane scaffold CD82 regulates cell adhesion by altering α4 integrin stability and molecular density. Mol Biol Cell. 25(10):1560-73.
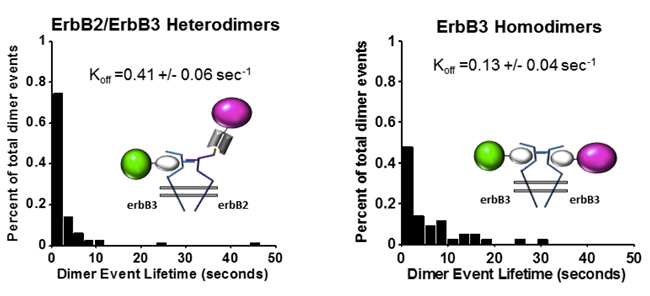
ErbB3 participates in homo- and hetero-interactions
ErbB3 was once thought to be a pseudokinase, dependent solely on heterodimerization with active kinases for phosphorylation. In this article, STMC investigators reveal that isolated erbB3 has ligand-dependent kinase activity that can be abolished by mutating a lysine in the erbB3 kinase domain. While erbB3 activation required interaction with erbB2, only minimal amounts of erbB2 were sufficient. Two color single particle tracking was performed of erbB2 and erbB3 receptors on the surface of live cells. Data revealed that erbB3 participates in transient homo-interactions, as well as hetero-interactions with erbB2. The significantly longer dwell time of homo-interactions supports a revised model that includes signaling competent erbB3 homodimers.
Mara P. Steinkamp, Shalini T. Low-Nam, Shujie Yang, Keith A. Lidke, Diane S. Lidke, and Bridget S. Wilson (2014) ErbB3 is an active tyrosine kinase capable of homo- and hetero-interactions. Mol Cell Biol. 34(6):965-977. PMID:24379439
Spatial Stochastic Modeling of EGFR/ErbB1 Dimerization
The dynamic nature of erbB1 receptor dimerization has been captured by single particle tracking methods developed by STMC investigators (Lidkes and trainees; see NSMB highlight below). In this mathematical modeling study, dimer off-rates measured on live cells were used as parameters for a spatial stochastic model of erbB1 dimerization kinetics. The model reveals the rapid transition states of erbB1 monomers as they diffuse, bind, and rebind after ligand addition. The model also provides insight into the complex interplay between interacting liganded and nonliganded species and tracks phosphorylation states of receptors as their kinase domains form asymmetric dimers. The model explores the influence of membrane domains and receptor abundance on the probability for dimerization to occur.
Pryor MM, Low-Nam ST, Halasz AM, Lidke DS, Wilson BS and Edwards JS. (2013) Dynamic Transition States of ErbB1 Phosphorylation Predicted by Spatial Stochastic Modeling. Biophysical Journal 105: 1533-1543.
Imaging Endocytosis in 3D
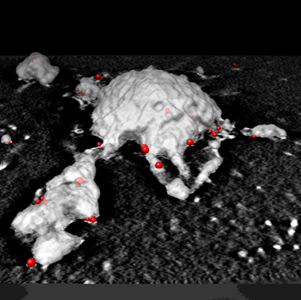
This publication from STMC investigators reveals the remarkable spatial relationships of receptors, clathrin, dynamin, Arf6 and Eps-15 positive structures on plasma membranes isolated from mast cells. In addition to applying our established 2D imaging techniques to study immunogold-labeled membrane sheets by transmission electron microscopy, electron tomography (at the Burnham Institute) is employed to capture the 3D topology of the inner surface of the plasma membrane as receptors are endocytosed. The study provides evidence for multiple specialized domains for endocytosis and reveals their close proximity. Intersections between specialized domains may represent sorting stations to direct cargo to specific endocytic pathways and/or the use of shared components.
View Cedric Cleyrat's ePoster Prezi slides from 2013 ASCB
Cleyrat C, Darehshouri A, Anderson KL, Page C, Lidke DS, Volkmann N, Hanein D, Wilson BS. (2013) The architectural relationship of components controlling mast cell endocytosis. Journal of Cell Science 126:4913-25
Modeling Ovarian Tumor Metastatic Spread in the Peritoneum
This study is based upon a xenograft model for ovarian cancer, where fluorescent human tumor cells were injected into the peritoneum of immuno-compromised mice. Tumor growth and spread were monitored by intra-vital imaging. Electron microscopy methods were also used to acquire high resolution information about local attachment sites, supporting the concept that the architecture of diverse organs in the peritoneal cavity underlie the differential growth and morphology of tumors attached to omentum, mesentary, spleen and gut. Experimental measures were used to build a 3D cellular Potts model that examines ovarian cancer cell attachment, chemotaxis, growth and vascularization. Image from the paper is featured on the cover at left.
Steinkamp MP, Winner KK, Davies S, Muller C, Zhang Y, Hoffman RM, Shirinifard A, Moses, M, Jiang Y and Wilson BS. (2013) Ovarian tumor attachment, invasion, and vascularization reflect unique microenvironments in the peritoneum: insights from xenograft and mathematical models. Frontiers in Oncology Vol 3, article 97.
*This article was featured in Frontiers as one of the Top 10 most viewed Oncology research articles in 2013.
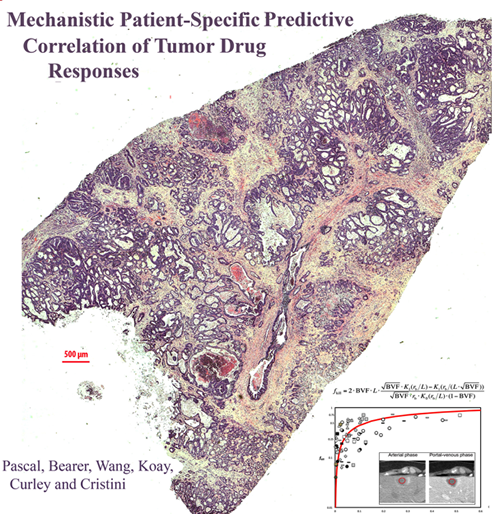
Modeling Tumor Drug Responses
This PNAS publication continues the Bearer-Cristini collaboration investigating mathematical modeling for prediction of tumor behavior by comparison to histopathology in human cancer specimens. The Bearer-Cristini team was supported by post-doc Jen Pascal, UNM research assistant professor Bill Wang and MD Anderson Cancer Center collaborators Curley and Kuay. They showed that a simple mathematical equation for diffusion can be used to predict chemotherapeutic response/delivery in tumors as diverse as brain tumors and colorectal cancer metastatic to liver. The paper has received significant media attention.
Pascal, J*, Bearer, EL*, Wang ZH, Kuay, EJ, Curley SA, Cristini, V. (2013) Diffusion Properties Limit Effectiveness of Chemotherapy in Metastatic Colorectal Cancer. Proceedings of the National Academy of Sciences USA Aug 12
*Authors shared equally
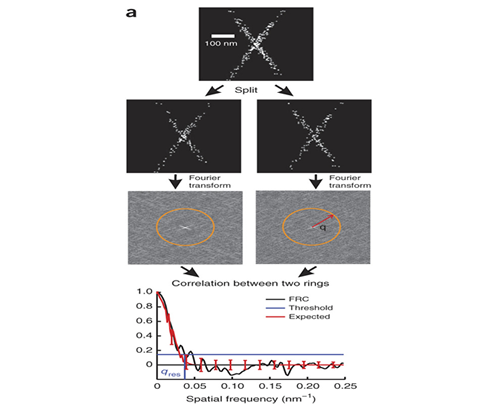
Measuring Image Resolution in Optical Nanoscopy
Single molecule localization based super-resolution techniques can provide images with resolution several times better than the diffraction limit. However, the achieved image resolution in a particular experiment is difficult to quantify because it cannot be given by specifications of the instrument and is instead a complicated function of the localization density, localization error, and the sample itself. Together with the Quantitative Imaging group at Delft (PI Bernd Rieger) we developed a method for using Fourier Ring Correlation to quantify image resolution for these methods.
R. P. J. Nieuwenhuizen, K. A. Lidke, M. Bates, D. L. Puig, D. Grünwald, S. Stallinga, and B. Rieger. (2013) "Measuring image resolution in optical nanoscopy.," Nature methods 10, 557–562 .
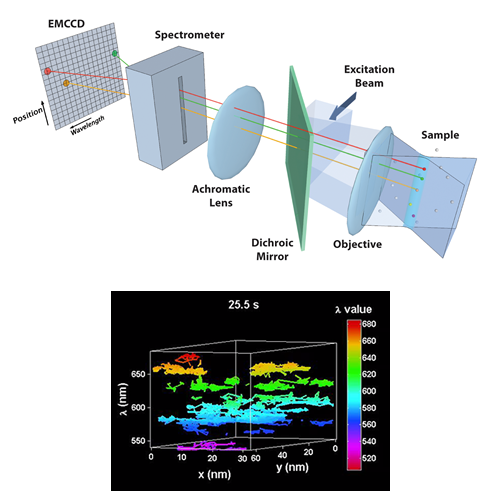
Multi-Color Quantum Dot Tracking Using a High-Speed Hyperspectral Line-Scanning Microscope
We have developed a novel high-speed hyperspectral microscope (HSM) to perform single particle tracking of up to 8 spectrally distinct species of quantum dots (QDs) at 30 frames per second. The distinct emission spectra of the quantum dots allows localization with ~ 10 nm precision even when the probes are clustered at spatial scales below the diffraction limit. The HSM is now being used to study the dynamics and stoichiometry of several types of membrane signaling complexes.
Cutler PJ, Malik MD, Liu S, Byars JM, Lidke DS, et al. (2013) Multi-Color Quantum Dot Tracking Using a High-Speed Hyperspectral Line-Scanning Microscope. PLoS ONE 8(5): e64320. doi:10.1371/journal.pone.0064320
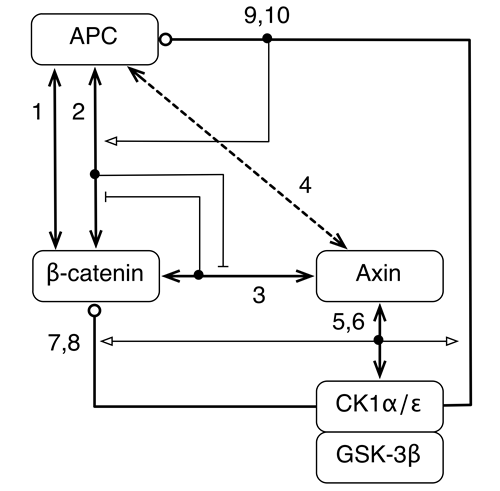
Effect of APC truncation on β-catenin destruction complex function
We asked the question, how can the effects of APC truncation, a very common mutation in colorectal cancer, be understood and reversed? We addressed this question by formulating a computational model for destruction complex function that incorporates site-specific details about protein-protein interactions and protein phosphorylation and examined the differences in predicted behaviors when APC is full length, as in normal cells, and truncated, as in colorectal cancer cells. Our model offers an explanation for how and why destruction complex function is altered by APC truncation. The model indicates that phosphorylation of the first 20-amino acid repeat in APC (which is usually the only 20-amino acid repeat that remains in truncated forms of APC) together with the absence of SAMP repeats (missing entirely because of truncation) allows truncated APC to act as a diversion sink. In other words, phosphorylated APC can outcompete Axin for binding to β-catenin, provided Axin is limiting, and thereby prevent β-catenin from associating with Axin and the Axin-associated kinases CK1α and GSK-3β, which initiate phosphorylation-depedent degradation of β-catenin. Thus, the model identifies inhibition of APC phosphorylation, which is mediated by CK1ε, as a potential means by which the oncogenic effect of APC truncation could be reversed.
Barua D, Hlavacek WS. (2013) Modeling the effect of APC truncation on destruction complex function in colorectal cancer cells. PLoS Computational Biology, in press.
Cargo-Motor Interactions for Fast Axonal Transport
This publication was in part a product of the Grand Challenge awarded by the STMC in spring 2012 to Cristini and Bearer. Using engineered fluorescent nanospheres, the group identified, quantitated and mathematically modeled the dynamics of transport of different cargo within a living axon. The results show that cargo-motor receptors regulate efficiency, accuracy, and speed of cargo delivery at long distances.
Seamster PE, Loewenberg M, Pascal J, Chauviere A, Gonzales A, Cristini V, Bearer EL. (2012) Quantitative measurements and modeling of cargo-motor interactions during fast transport in the living axon. Phys Biol. 2012 Oct;9(5):055005.
ErbB1 dimerization is promoted by domain co-confinement and stabilized by ligand
Ligand-induced signaling by the epidermal growth factor receptor (EGFR or HER1 or erbB1) drives cell growth and survival, with roles in normal development and disease pathogenesis. A wealth of structural knowledge supports a model of signal initiation through the formation of back-to-back erbB1 dimers. However, conclu¬sions about the size and ligand-occupancy of the erbB1 signaling complex remain controversial. We used two-color single quantum dot tracking to directly observe and quantify erbB1 homodimerization on living cells. To quantify the kinetics of dimerization, a 3-state Hidden Markov Model was developed to extract transition rates between free, co-confined, and dimer states. It was found that 2 ligand-bound receptors form more stable dimers than resting receptors, linking ligand occupancy to dimer stability. Furthermore, actin-based confinement was found to promote receptor dimerization.
S.T. Low-Nam, K.A. Lidke, P.J. Cutler, R.C. Roovers, P.M.P. van Bergen en Henegouwen, B.S. Wilson, D.S. Lidke. (2011) "ErbB1 dimerization is promoted by domain co-confinement and stabilized by ligand." Nature Structural & Molecular Biology. 18:1244-1249
Small, mobile FcεRI aggregates are signaling competent
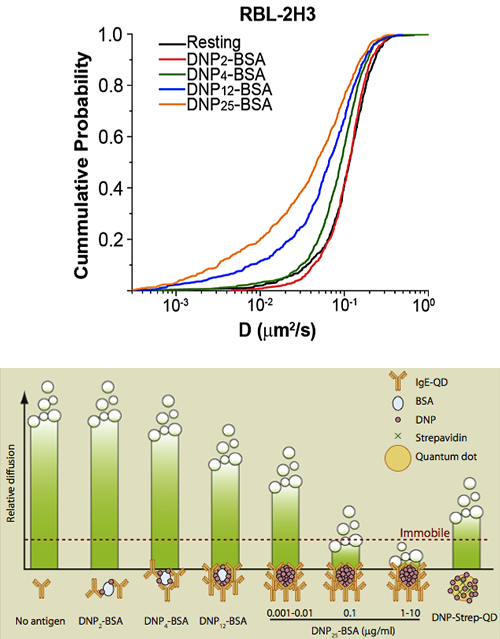
Crosslinking of IgE-bound FcεRI triggers mast cell degranulation. Previous studies suggested that FcεRI must immobilize to induce signal. We used single quantum dot (QD) tracking and hyperspectral microscopy methods to define the relationship between receptor mobility and signaling. QD-IgE-FcεRI aggregates of at least three receptors remained highly mobile over extended times at low concentrations of antigen that induced Syk kinase activation and near-maximal secretion. FcεRI immobilization was marked at intermediate and high antigen concentrations, correlating with increases in cluster size and rates of receptor internalization. We propose that immobility is a feature of highly crosslinked immunoreceptor aggregates and a trigger for receptor internalization, but is not required for tyrosine kinase activation leading to secretion.
See N.L. Andrews, J.R. Pfeiffer, A.M. Martinez, D.M. Haaland, R.W. Davis, T. Kawakami, J.M. Oliver, B.S. Wilson and D.S. Lidke. (2009) "Small, mobile FcεRI aggregates are signaling competent." Immunity, 31: 469-479
Also covered in Immunity Previews, 31, September 18, 2009 entitled Hierachy of Diffusion Behavior. The figure at left had this caption: "Shown are relative diffusion coefficients of different kinds of antigens used by Andrews et al. in this issue of Immunity. Signaling complexes initiated by single antigen molecules exhibit substantial diffusion (DNP2-BSA, DNP4-BSA, DNP12-BSA, DNP-Strep-QD, and 0.001–0.01 mg/ml of DNP25-BSA); however, larger complexes generated by higher concentrations of DNP25-BSA undergo arrest. These experiments reveal a hierarchy of diffusion behavior that correlates directly with size of complexes formed."
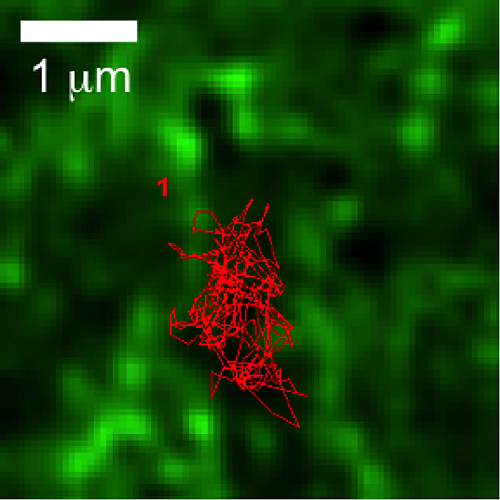
Actin restricts FcεRI diffusion and facilitates antigen induced immobilization
The concept of actin "confinement zones" has been proposed for more than a decade. Through simultaneous observations of quantum dot (QD)-labeled FcεRI motion and GFP-tagged actin dynamics, we provided the first direct evidence for actin barriers that influence protein diffusion on the time scale of seconds and length scale of microns. Dynamic reorganization of actin structures occurs over seconds, making the location and dimensions of actin-defined domains time dependent. We also designed a novel assay to quantify receptor immobilization kinetics in response to cross-linking by multivalent antigen. This assay revealed that dramatic changes in receptor mobility occur within seconds of antigen binding and the kinetics are dependent upon an intact actin cytoskeleton. These new insights not only confirm that actin acts as a diffusion barrier, but also demonstrate a significant role for actin in modulating protein-protein interactions and ligand binding response.
N.L. Andrews, K.A. Lidke, J.R. Pfeiffer, A.R. Burns, B.S. Wilson, J.M. Oliver and D.S. Lidke. (2008) "Actin restricts FcεRI diffusion and facilitates antigen induced immobilization." Nature Cell Biology, 10:955-963